


Conducting GMP Auditing Services Worldwide: We conduct high standards of GMP audits of different types of suppliers across the globe as per different regulatory standards (i.e. EU GMP, ICH Q7, 21 CFR 210/211, ISO etc. for GMP) as requested by the clients; we also perform the tailor made/customized audits as per the customer’s requirement including specific focused areas, specific standards, specific product/system, criticality or even for cause audits.
Our audit reports are very detailed and top quality hence well accepted globally by regulators, QPs, subject matter experts etc. We conduct GMP audits of following different types of suppliers, but not limited to:
API / API intermediates; sterile/non-sterile
Formulated medicinal products; sterile/non-sterile
Biological, fermentation-based APIs and formulation products
Excipients / Key Starting Materials (KSMs)/ raw materials/ cosmetics
Contract testing Laboratories
Different services providers
Distributors; GDP
Packaging materials
Components used in the pharmaceutical industries
Processing sites (i.e. micronization, sterilization, radiation etc.)

Quality GMP Auditing Services: Depending upon customers’ request, our Quality auditing service can start from initiating the audit with the supplier upto providing complete AUDIT REPORT PACKAGE to our client which includes all the below steps:
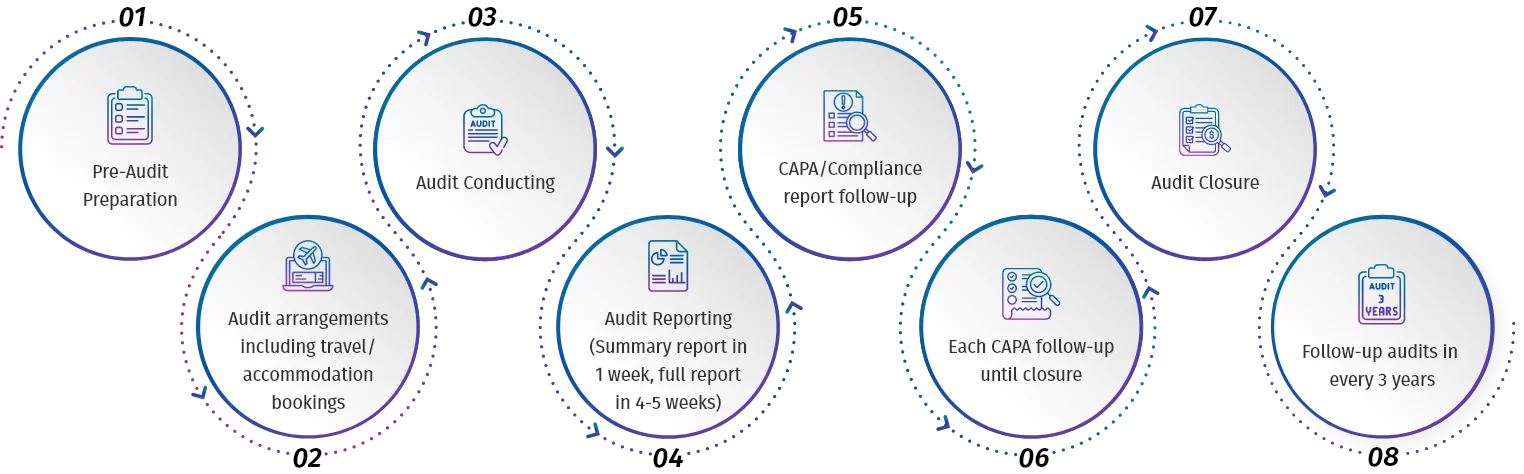
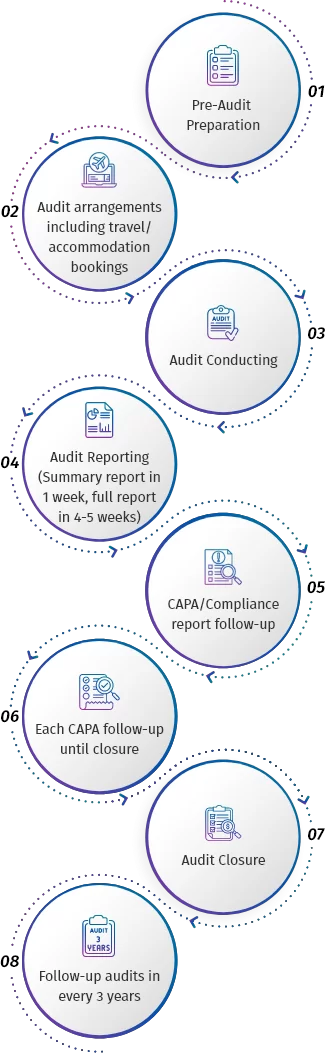
Our Audit Report Package is very thorough and includes all of the following which is sufficient enough to qualify the supplier: